Abstract
Introduction Multiple myeloma (MM) is a cancer of older adults with significant morbidity and mortality. Frail patients are a high-risk subgroup at risk for poor outcomes. While a number of tools have been developed for frailty assessment, many are measured only at baseline and do not take into account changes in frailty over time. Understanding both improvements and deteriorations in frailty over the disease trajectory may have important considerations in modifying treatment delivery based upon a patient's changing fitness status.
Electronic frailty indices using a cumulative deficit approach have emerged as an important tool for identifying frail older adults in clinical practice through the electronic medical record. A cumulative deficit approach allows for characterization of frailty by evaluating an individual's health status as a proportion of ageing-associated deficits an individual has incurred. This approach may allow for a more dynamic measurement of frailty as 'deficits' may drop off when not actively coded or new 'deficits' may accumulate with increasing co-morbidities. Using an administrative database and the cumulative deficit approach to categorizing frailty, we aimed to 1) evaluate the dynamic trajectory of frailty among a cohort of older adults with MM during the first three years following diagnosis 2) identify factors associated with improvements or deterioration of frailty over time.
Methods We conducted a retrospective population-based study of adults with MM (ICD-O-3 code 9732) using the SEER-Medicare linked database. Inclusion criteria included MM patients aged 66 or older treated with novel agents in first line between 2007-2014. Frailty was defined according to a previously validated frailty index based on the cumulative deficit definition (Cheung et al. J Gerontol A Biol Sci Med Sci, 2021). An annual frailty index (possible range 0-1) was calculated using 31 age-related health deficits, identified using diagnostic and procedural codes. Variables used to calculate the frailty index for a given year were drawn from the prior 12 months. Patients were categorized into five pre-defined groups: nonfrail (0-0.10), prefrail (0.11-0.20), mildly frail (0.21-0.30), moderately frail (0.31-0.40) and severely frail (>0.4). A multivariate cox-regression model was conducted at baseline to assess the association of frailty with overall survival (OS) both at baseline and at each landmark interval.
Results A total of 4617 patient with MM were identified. Baseline characteristics are shown in Table 1. At baseline, 39% of the patients were categorized as moderately frail or severely frail. In multivariate analysis, older age, female gender, black race, and Medicaid enrollment were associated with higher frailty status. Immunomodulatory drug adminstration during first-line treatment was lower among patients with higher frailty status.
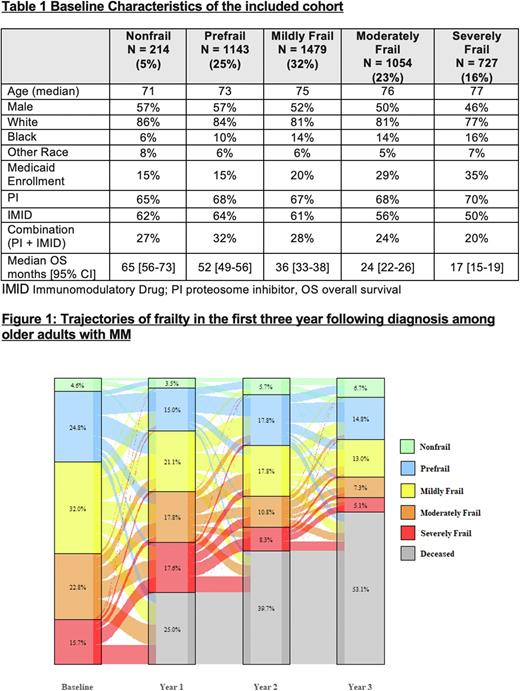
There was a clear gradient effect of frailty on OS; the median OS for nonfrail patients was 65 months [95% CI 56-73] compared to 17 months [95% CI 15-19] for severely frail. Compared to nonfrail patients, the mildly frail had a 60% increase in hazard for death, the moderately frail a 104% increase, and the severely frail a 165% increase after controlling for other covariates (all p <0.001). The predictive ability of baseline frailty for OS decreased over time in landmark analyses (Harrell's C Statistic 0.65 at diagnosis, 0.63 at 1 yr, 0.62 at 2 yr, and 0.60 at 3 yr). Trajectories of frailty from baseline over the next three years is shown in Figure 1.
One year following diagnosis, 15% of the patients showed in an improvement in the frailty status, 33% had a deterioration, 26% remained the same, and 25% had died. On multivariate analysis, older age, male gender, and Medicaid enrollment were associated with increased odds of worsening frailty status at one year. In landmark analyses at years 1, 2 and 3 post diagnosis, the predictive ability of current frailty status remained fairly consistent (Harrell's C Statistic 0.64-0.65).
Conclusion Our study is one of the first to demonstrate the dynamic nature of frailty among older adults with MM. Frailty can both improve and deteriorate over time. Current frailty status is a better predictor of outcomes than baseline frailty status, indicating the need for re-measurement. Future strategies aimed at further assessing and incorporating dynamic frailty monitoring over time are needed to provide more tailored treatment.
Disclosures
Mian:GSK, Janssen, BMS/Celgene, Forus therapeutics, Amgen, Takeda, Sanofi: Honoraria; Janssen: Research Funding. Wildes:Janssen: Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees; Carevive, Sanofi: Consultancy. Vij:Adaptive: Honoraria; Sanofi: Honoraria, Research Funding; GSK: Honoraria; Pfizer: Honoraria; BMS: Honoraria, Research Funding; Legend: Honoraria; Biegene: Honoraria; Janssen: Honoraria; CareDx: Honoraria; Harpoon: Consultancy; Oncopeptides: Honoraria; Takeda: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal